
Complete Guide Medical Device Classification EU MDR (Free PDF)
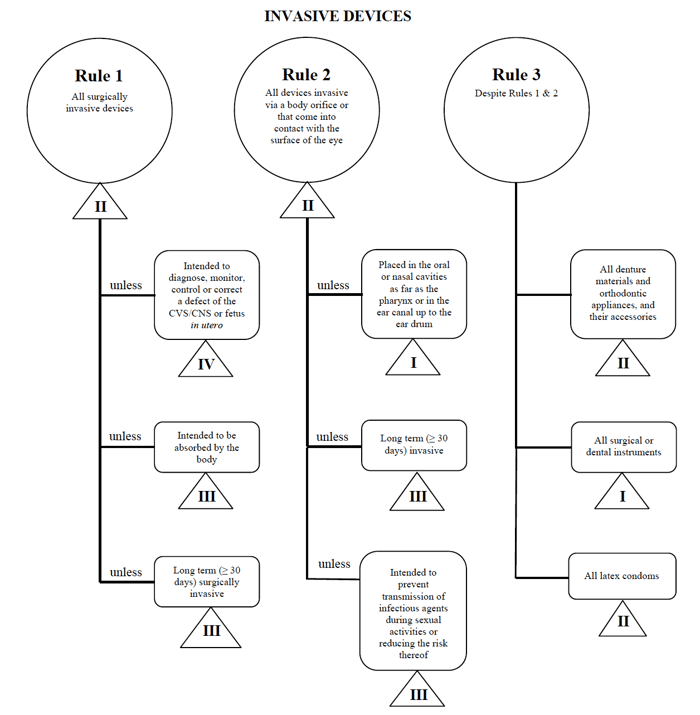
European Union's Regulation 2017 /745 on medical devices, for instance, states that "'invasive device' means any device. which, in whole or in part, penetrates inside the body, either.

Medical device Patients can walk again only 90 minutes after invasive hip surgery FHI
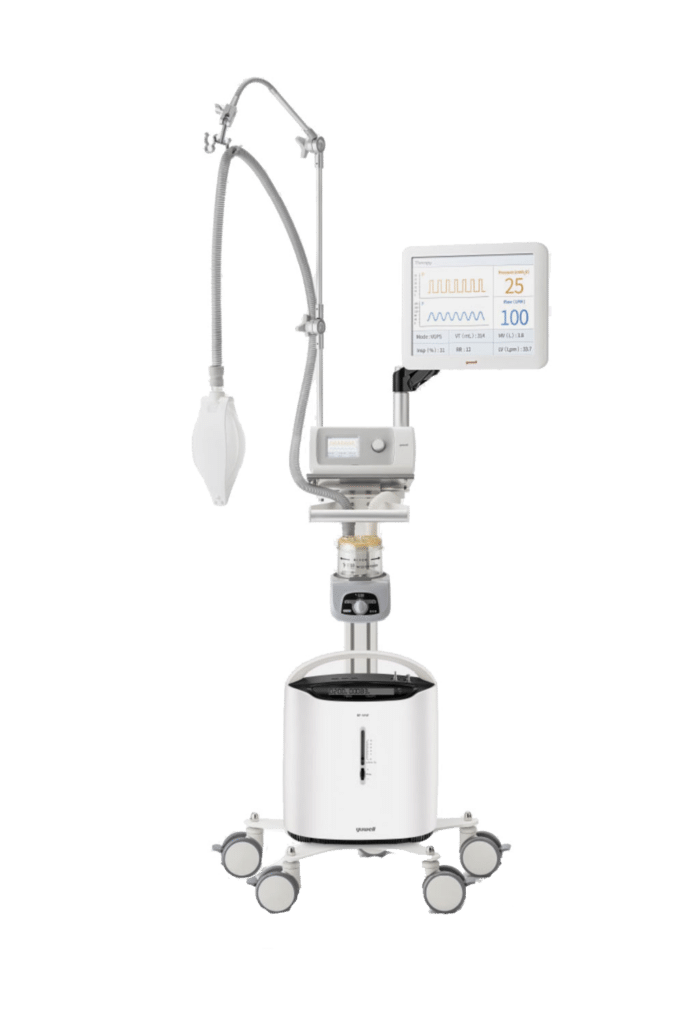
Infections can be associated with the devices used in medical procedures, such as catheters or ventilators. These healthcare-associated infections (HAIs) include central line-associated bloodstream infections, catheter-associated urinary tract infections, and ventilator-associated pneumonia. Infections may also occur at surgery sites, known as.
FDA Guidance on Medical Device Patient Labeling Overview RegDesk
ter understanding of what makes a medical device invasive. 4 Invasiveness of medical devices As far as I can discern, invasiveness has no distinct or con-sensus meaning in medicine or biomedical science. Regu-latory agencies do make reference to invasiveness. The European Union's Regulation 2017/745 on medical devices, for instance, states.

Invasive devices increase risk of infection The BMJ
The use of invasive devices to deliver short-term treatments, relief of problems such as urinary retention, and long-term therapies has revolutionised healthcare. Invasive devices breach the normal defences of the body and therefore provide potentially easy access for micro-organisms.1 In addition to their widespread use as part of in-patient.
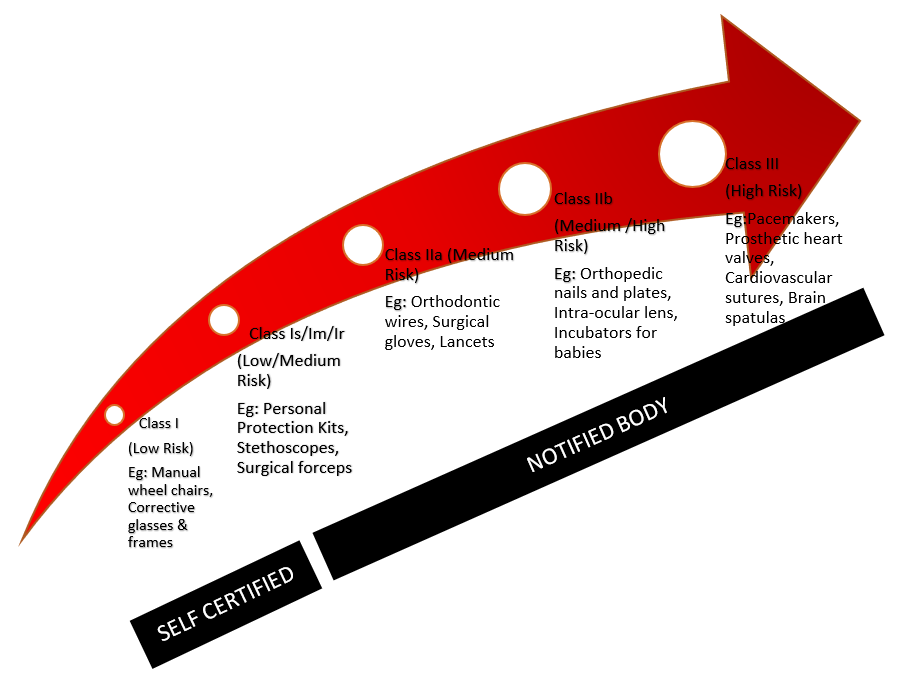
Medical Device Classification Guide How To Determine Your Device Class (2022)
Abstract. Medical devices are often referred to as being invasive or non-invasive. Though invasiveness is relevant, and central, to how devices are understood and regarded in medicine and bioethics, a consensus concept or definition of invasiveness is lacking. To begin to address this problem, this essay explores four possible descriptive.

Types and sites of invasive procedures or surgery. Minor and major... Download Scientific Diagram
knee, hip or shoulder. joint replacement implants. motion‑preserving device for the spine. (such as a spinal disc replacement) intended for contraception or prevention of sexually transmitted diseases and that. are implantable. or invasive for long-term use. active implantable medical devices. implantable.

Minimally Invasive Devices Floshield Priority Designs
The concept of medical invasiveness, as some have called it (Rudnick 2011 ), is a feature of biomedical, regulatory, and bioethics discourses about medical devices. 1 Medical devices, like pacemakers, intrauterine devices (IUDs), electrocardiograms (EKGs), and many others are commonly referred to as invasive or non-invasive (or minimally.

Medical Devices; US and Chinese legislation Kvalito
An invasive medical device is one that is intended by the manufacturer to be used, in whole or in part, inside the body of a human being. This includes devices that enter a body orifice or that penetrate the surface of the body, or that enter the body in the context of a surgical operation. A medical device that touches the surface of the eye.
i3cglobal Medical Device Classification
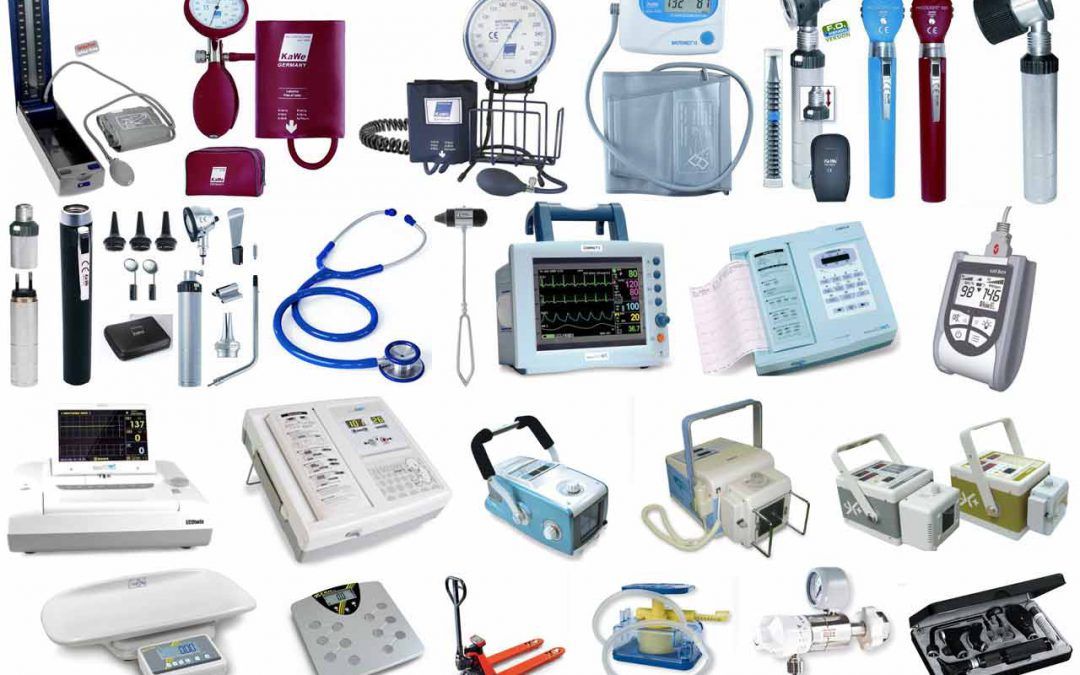
An invasive device is any medical device introduced into the body. They enter either through a break in the skin or an opening in the body. Examples of common invasive devices include: Urinary catheters: Urinary catheters are rubber or silicone tubes inserted through your urethra to your bladder. A small balloon at the tip of the catheter is.

Medical Device Regulations. Design Requirements PresentationEZE
HAIs are considered an undesirable outcome, and as some are preventable, they are considered an indicator of the quality of patient care, an adverse event, and a patient safety issue.. at least 90 percent of infections were associated with invasive devices. 23 Invasive medical devices bypass the normal defense mechanism of the skin or mucous.
Invasive and NonInvasive Medical Equipment
Biomedical devices provide a critical role in the healthcare system to positively impact patient well-being. This paper aims to provide the current classifications and subclassifications of hardware and software medical devices according to the Food and Drug Administration (FDA) guidelines. An overview of the FDA regulatory pathway for medical device development such as radiation-emitting.
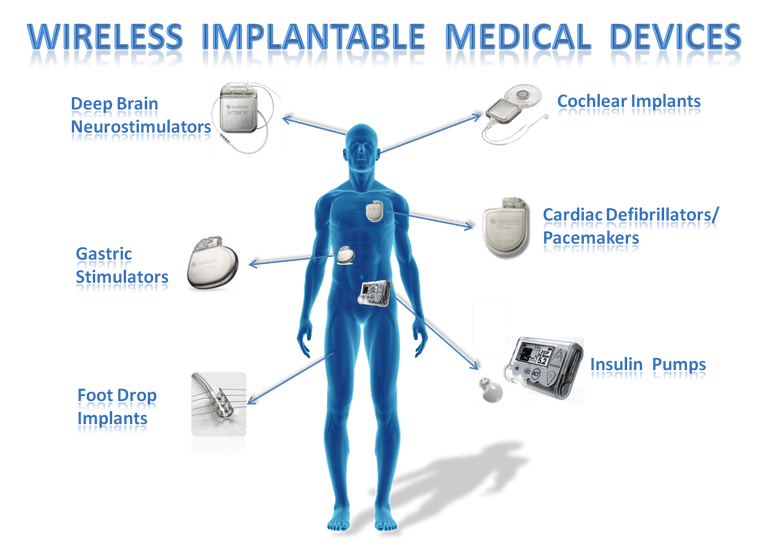
MultiFunction Chip Developed To Enable Next Generation Implantable Device Innovations Medical
2. HAIs Associated with Administration of Temporary Indwelling Devices. Several studies have identified that the kind of microorganisms causing an HAI depends on the type of medical implant inserted into the patient [17,20].Additionally, it is necessary to determine precisely whether the patient got the infection before admission to the hospital or became infected during hospitalization.

PPT Where the Microbes are Lurking Preventing Cross Contamination via Medical Devices
Based on past FDA 162 and Medtech Europe estimates, more than 500,000 types of medical devices have currently entered the global market.Invasive medical devices, including indwelling and.

Checking Invasive Devices Using Chest XRays Dr. GrepMed
The concept of medical invasiveness, as some have called it (Rudnick 2011 ), is a feature of biomedical, regulatory, and bioethics discourses about medical devices. 1 Medical devices, like pacemakers, intrauterine devices (IUDs), electrocardiograms (EKGs), and many others are commonly referred to as invasive or non-invasive (or minimally.
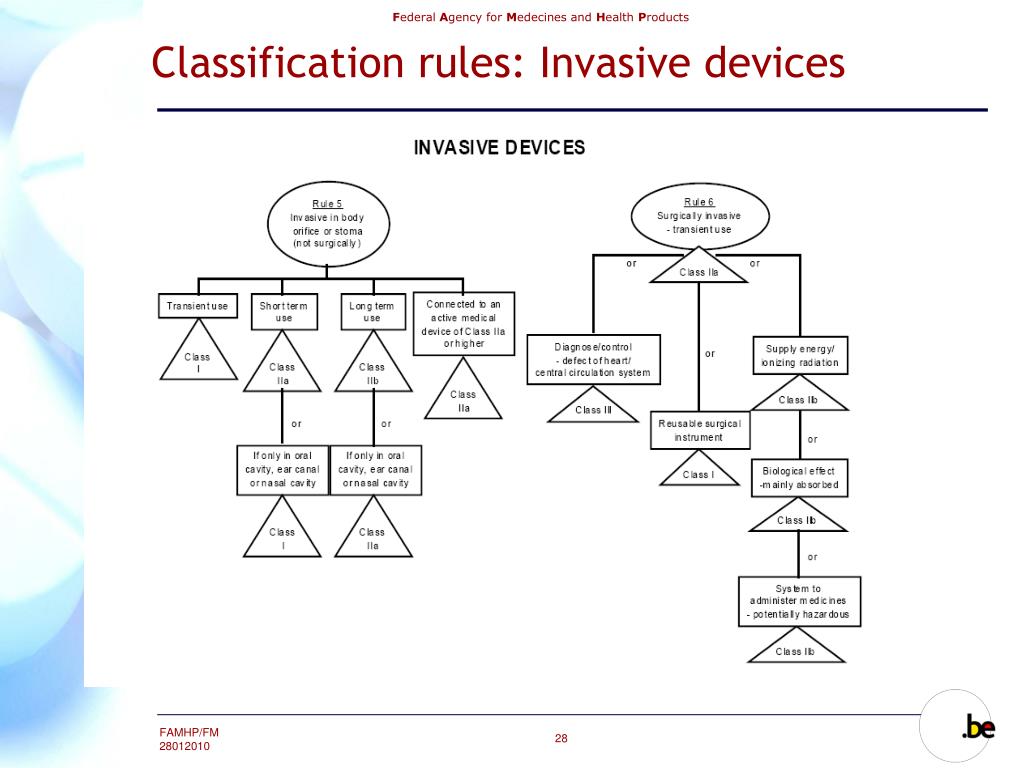
PPT Federal Agency for Medicines and Health Products PowerPoint Presentation ID6347669
Use an alcohol-based hand rub or wash with soap and water for the following clinical indications: Immediately before touching a patient; Before performing an aseptic task (e.g., placing an indwelling device) or handling invasive medical devices; Before moving from work on a soiled body site to a clean body site on the same patient
Invasive and NonInvasive Medical Equipment
Abstract. The present study evaluated the daily risk of healthcare-associated infections and sepsis (HAIS) events in pediatric intensive care unit patients with invasive devices. This was a retrospective cohort study. Invasive devices were associated with significant daily risk of HAIS ( p < 0.05). Endotracheal tubes posed the greatest risk of.
- St Kilda Football Club Membership
- Italian Restaurants Hyde Park Chicago
- Bull Arab Cross Bull Mastiff
- Florence Italy To Cinque Terre
- Toheeb Jimoh Movies And Tv Shows
- Always With Me Piano Sheet
- Things To Do In Rotterdam Holland
- 5 Letter Word Ending In Ge
- Royal Australian Corps Of Transport
- Brisbane Roar Vs Western Sydney Wanderers Fc Stats