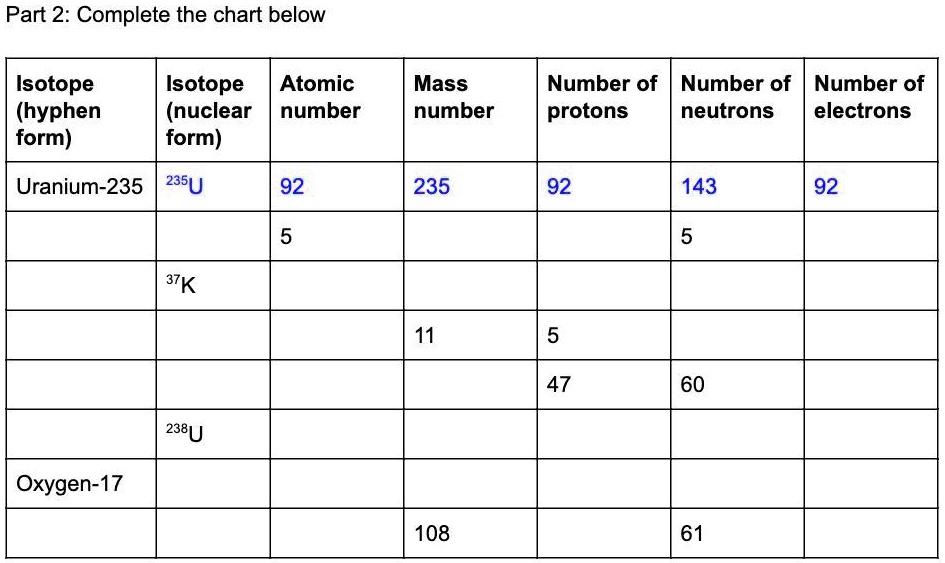
SOLVED Please fill out correctly 15 POINTS Part 2 Complete the chart below Isotope (hyphen
78. 77.96318 (118)#. Mass Number. The sum of the number of protons and neutrons of an atomic nucleus. In other words, it's the sum of the number of nucleons in an atom. Relative Atomic Mass. The ratio of the average mass per atom of an isotope to 1/12 the mass of a carbon-12 atom.
PPT to Microbiology! PowerPoint Presentation, free download ID5323535
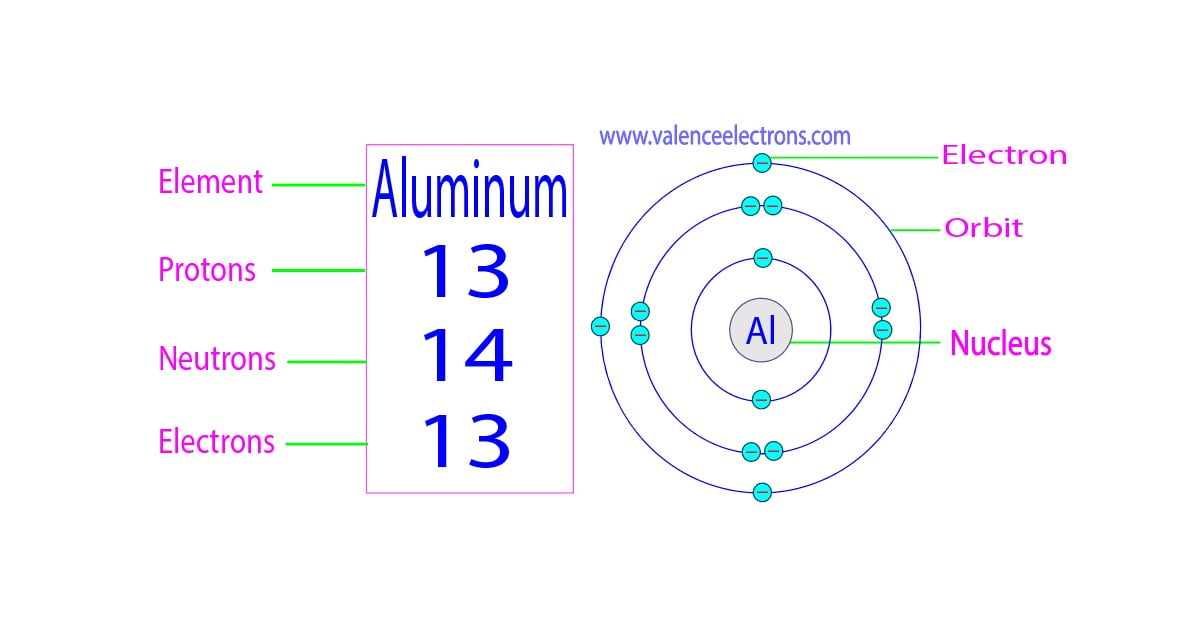
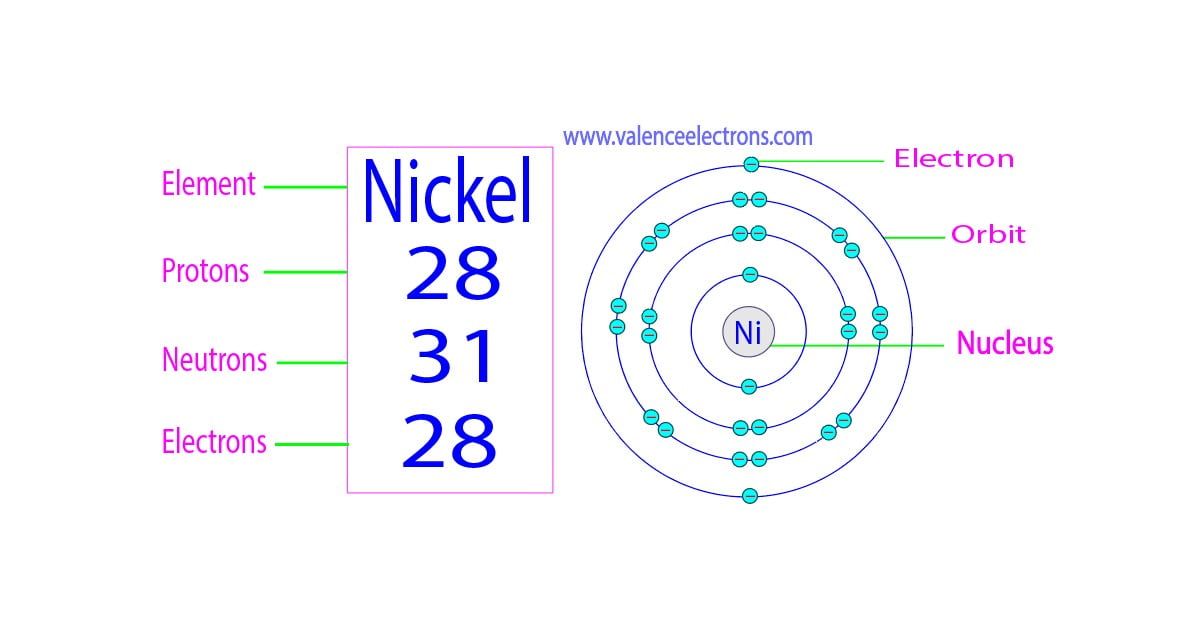
Atomic number (number of protons in the nucleus): 28; Atomic symbol (on the periodic table of the elements): Ni; Atomic weight (average mass of the atom): 58.6934; Density: 8.912 grams per cubic.
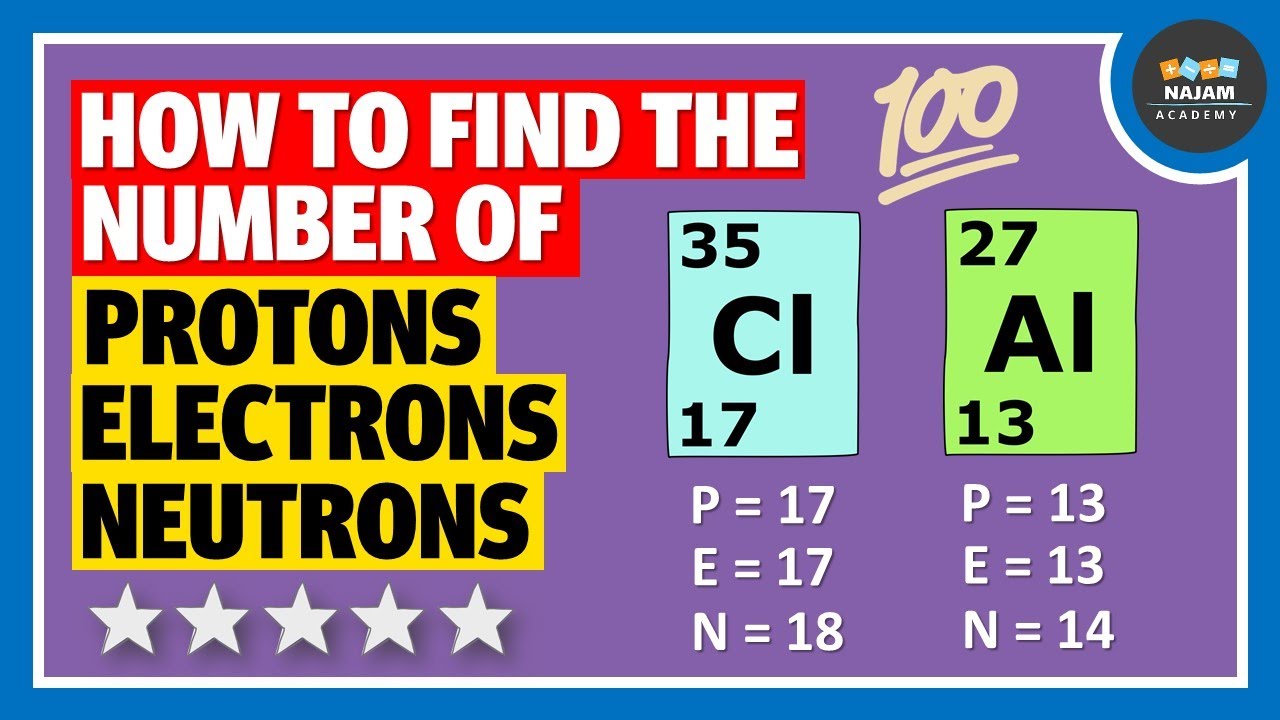
How to find the number of Protons, Neutrons and Electrons? Chemistry YouTube
Number of Neutrons in Nickel. The number of neutrons can be found by subtracting the atomic number from its atomic mass. Number of Neutrons in Nickel = Atomic mass of Nickel - Atomic number of Nickel = 59 - 28 = 31. Number of Electrons in Nickel. For a neutral atom, the number of electrons can be found by knowing the atomic number of that atom.

How To Calculate The Number Of Protons Neutrons And E vrogue.co
Make sure that you round the atomic mass to the nearest whole number. For example, the atomic mass of boron is 10.811, but you can just round the atomic mass up to 11. 6. Subtract the atomic number from the atomic mass. To find the number of neutrons, you will need to subtract the atomic number from the atomic mass.
Periodic Table Number Of Protons
Nickel is a chemical element;. As a compound, nickel has a number of niche chemical manufacturing uses,. With 28 protons and 20 neutrons, 48 Ni is "doubly magic", as is 78 Ni with 28 protons and 50 neutrons. Both are therefore unusually stable for nuclei with so large a proton-neutron imbalance.
Nickel Unites Protons Rapidly
Atomic Number - Protons, Electrons and Neutrons in Nickel. Nickel is a chemical element with atomic number 28 which means there are 28 protons in its nucleus.Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z.The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs.

Nickel Protons Neutrons Électrons Configuration électronique
The mass of the atom is a unit called the atomic mass unit (amu) ( amu). One atomic mass unit is the mass of a proton, or about 1.67 ×10−27 1.67 × 10 − 27 kilograms, which is an extremely small mass. A neutron has just a tiny bit more mass than a proton, but its mass is often assumed to be one atomic mass unit as well.
How many protons, neutrons and electrons does nickel have?
Number of Protons: 28: Number of Neutrons: 31: Number of Electrons: 28: Melting Point: 1453.0° C: Boiling Point: 2732.0° C: Density: 8.902 grams per cubic centimeter: Normal Phase:. The first nickel coin of the pure metal was made in 1881. Common Uses: Coinage in the United States and Canada; Stainless steel; Corrosion-resistant alloys;
PPT How many protons , electrons, and neutrons are in an atom? PowerPoint Presentation ID
Protons, Neutrons, Electrons for Nickel (Ni2+, Ni3+) Nickel is a classified transition metal and its symbol is Ni. Nickel is the 28th element of the periodic table so its atomic number is 28. The atomic number of an element is equal to the number of protons and electrons in that element. Therefore, a nickel atom has twenty-eight protons and.

Symbol And Electron Diagram For Nickel Electron Chemical Clip Vector, Electron, Chemical, Clip
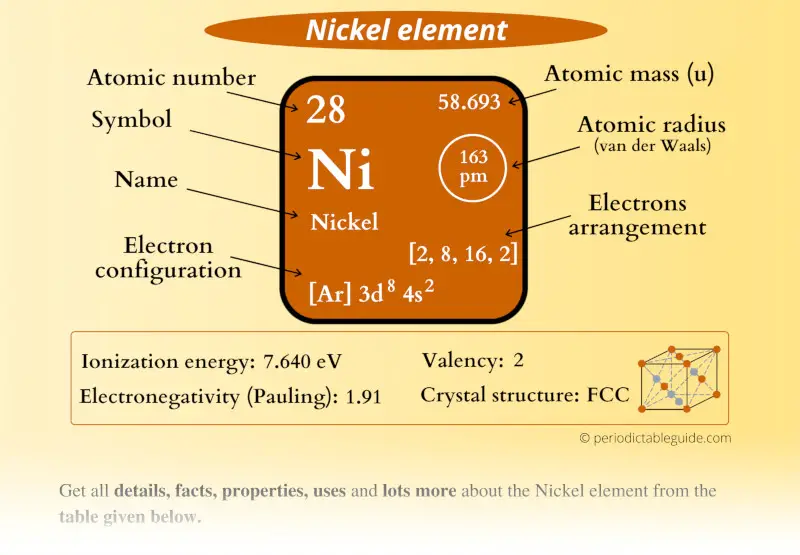
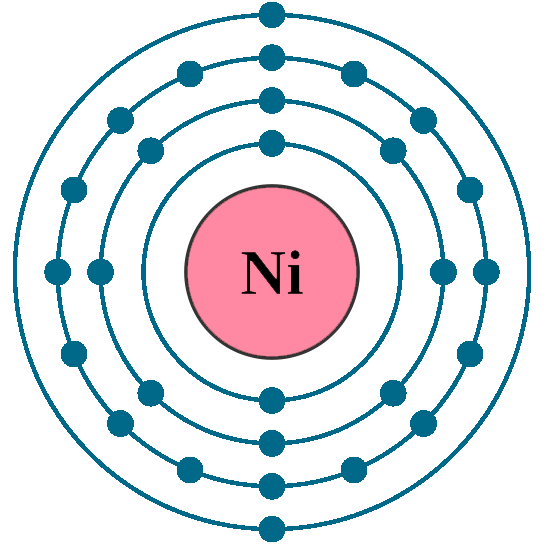
Nickel (Ni) Nickel is the 28th element in the periodic table and has a symbol of Ni and atomic number of 28. It has an atomic weight of 58.6934 and a mass number of 58. Nickel has twenty-eight protons and thirty neutrons in its nucleus, and twenty-eight electrons in four shells. It is located in group ten, period four and block d of the.
Nickel (Ni) Periodic Table (Element Information & More)
Name: Nickel Symbol: Ni Atomic Number: 28 Atomic Mass: 58.6934 amu Melting Point: 1453.0 °C (1726.15 K, 2647.4 °F) Boiling Point: 2732.0 °C (3005.15 K, 4949.6 °F) Number of Protons/Electrons: 28 Number of Neutrons: 31 Classification: Transition Metal Crystal Structure: Cubic Density @ 293 K: 8.902 g/cm 3 Color: white Atomic Structure

Nickel by fjh
Nickel's properties, discovery, videos, images, states, energies, appearance and characteristics.. Protons: 28: Neutrons in most abundant isotope: 30: Electron shells: 2,8,16,2 : Electron configuration:. the Swedish chemist Axel Cronstedt carried out a number of experiments to determine the true nature of kupfernickel. (We now know that.

Periodic Table Number Of Protons
Nickel is a chemical element of the periodic table with chemical symbol Ni and atomic number 28 with an atomic weight of 58.6934 u and is classed as a transition metal.. Number of protons: 28 p + Number of neutrons: 31 n 0: Number of electrons: 28 e-From Wikipedia, the free encyclopediaNickel is a chemical element with symbol Ni and atomic.
Protons, Neutrons, Electrons for Nickel (Ni2+, Ni3+)
In this video we'll use the Periodic table and a few simple rules to find the protons, electrons, and neutrons for the element Nickel (Ni). From the Periodic.
.PNG)
two atoms with the same number of protons but a different number of neutrons are
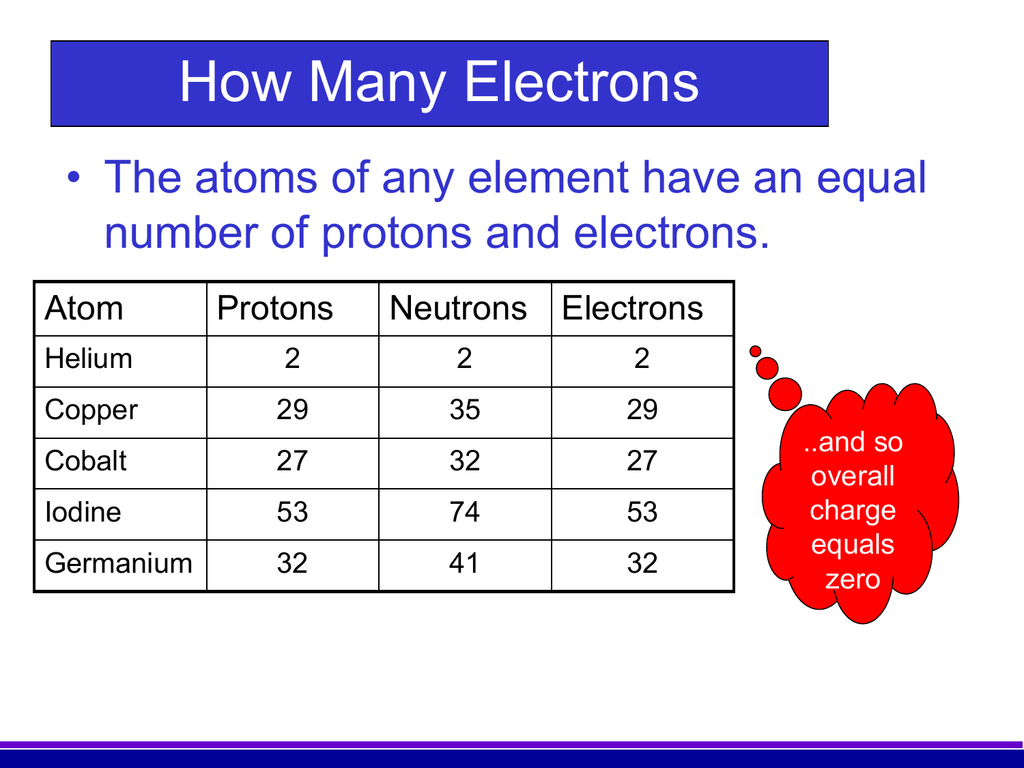
While protons and neutrons are located inside the nucleus at the center of the atom, electrons are located outside the nucleus in what is often called the electron cloud. Figure 4.4.1 4.4. 1: Electrons are much smaller than protons or neutrons. If an electron was the mass of a penny, a proton or a neutron would have the mass of a large bowling.
Nickel Periodic Table Charge Elcho Table
Atomic Number of Nickel. Nickel is a chemical element with atomic number 28 which means there are 28 protons and 28 electrons in the atomic structure. The chemical symbol for Nickel is Ni. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The nucleus is composed of protons and neutrons.
- Forrest Griffin And Stephan Bonnar
- Hotels In Fisherman S Wharf San Francisco Ca
- Short Hair Parted In The Middle
- I Can Walk 500 Miles Lyrics
- Rise Fall Civilizations At War
- Car Cigarette Lighter Power Adapter
- Forte School Of Music Stafford
- What Is The Fine For Not Voting In The Referendum
- Bob Hair Cut With Fringe
- Weather In March In Korea